20 Jan 2026
- 8 Comments
When a pill leaves the factory, it needs to stay safe and effective for months-or even years-before a patient takes it. That’s not magic. It’s stability testing. And at the heart of that process are two non-negotiable factors: temperature and time.
Why Temperature and Time Matter in Drug Stability
Imagine a bottle of medicine sitting in a hot warehouse in India, or a vaccine stored in a fridge that fluctuates between 2°C and 8°C. If the drug breaks down, it might not work. Worse, it could turn toxic. Stability testing is the system that catches this before it reaches you. The global standard, set by the International Council for Harmonisation (ICH), is built on decades of science. It doesn’t guess. It measures. And it demands exact conditions: specific temperatures, controlled humidity, and precise time intervals. This isn’t about bureaucracy. It’s about safety. In 2022 alone, the FDA issued 27 warning letters to drugmakers because their stability data didn’t meet requirements. Some of those led to recalls of hundreds of thousands of doses.The ICH Q1A(R2) Standard: The Global Baseline
The ICH Q1A(R2) guideline, finalized in 2003, is still the rulebook today. It applies everywhere-from the U.S. to the EU, Canada, Japan, and beyond. There are three main testing zones, each with its own temperature and humidity rules.- Long-term testing: This is the real-time clock. You store the drug under conditions that match where it’ll be sold. Two options exist: 25°C ± 2°C at 60% RH ± 5% RH, or 30°C ± 2°C at 65% RH ± 5% RH. The choice depends on the climate zone of the target market. For example, if you’re selling in Southeast Asia (Zone IVa), you must use the 30°C/65% RH condition. You need at least 12 months of data before you can submit the drug for approval in the U.S. The EU allows 6 months, but that can delay global launches.
- Accelerated testing: This is the stress test. All drugs, whether they’re tablets, capsules, or liquids, go through 40°C ± 2°C at 75% RH ± 5% RH for six months. This isn’t meant to be realistic-it’s meant to predict what will happen over years. If the drug fails here, you know you’ve got a problem. This test helps catch degradation early. For most small-molecule drugs, six months at 40°C mimics about two years at room temperature.
- Intermediate testing: This is the backup plan. If your long-term study is done at 25°C and the accelerated test shows a problem, you run a six-month test at 30°C/65% RH to confirm what’s happening. It’s not always required, but when it is, it can save a product from failure in tropical markets.
Refrigerated and Frozen Drugs: Different Rules
Not all drugs are stored at room temperature. Insulin, many vaccines, and biologics like monoclonal antibodies need cold storage. Their stability rules are different.- Refrigerated long-term: 5°C ± 3°C for 12 months. This is the standard for drugs that must stay cold from day one.
- Refrigerated accelerated: 25°C ± 2°C at 60% RH ± 5% RH for six months. Notice it’s not 40°C. That’s because freezing and thawing, not heat, are the real threats here.
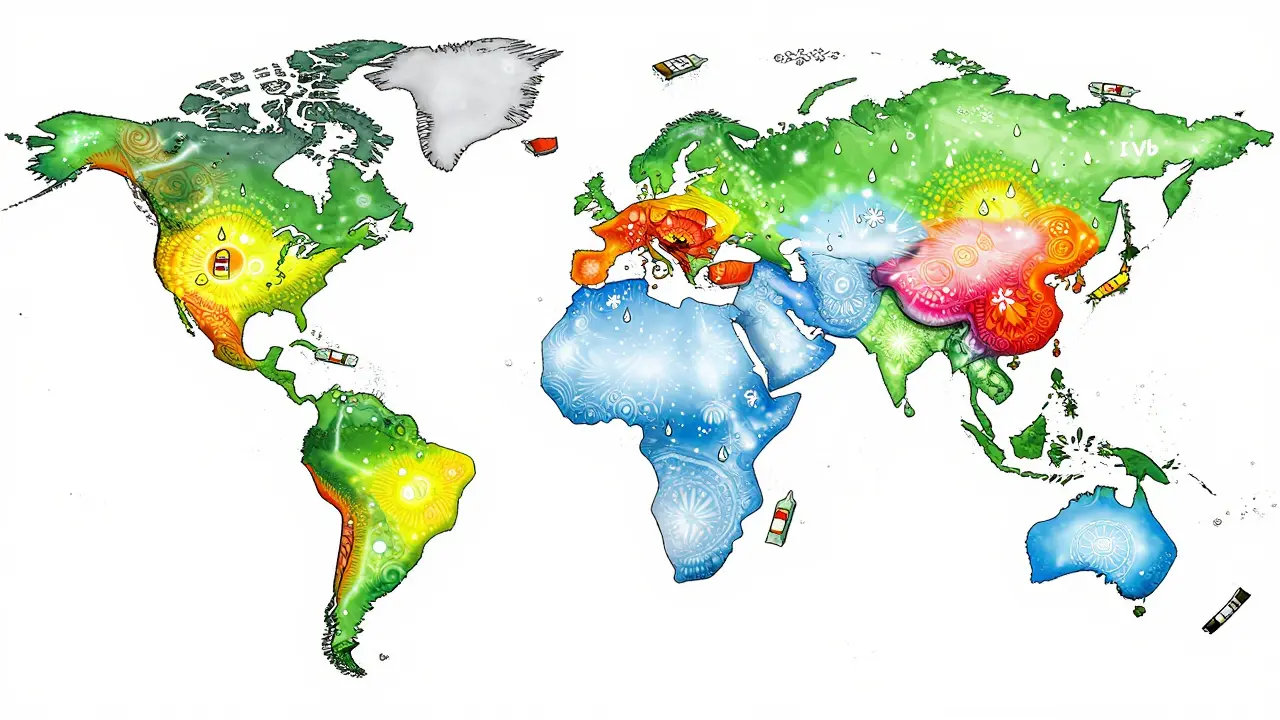
Global Climates, Different Rules
The world isn’t one climate. ICH breaks it into five zones:- Zone I (Temperate): 21°C / 45% RH - Think Europe, Canada, northern U.S.
- Zone II (Mediterranean/Subtropical): 25°C / 60% RH - Southern U.S., Australia, parts of China.
- Zone III (Hot-Dry): 30°C / 35% RH - Middle East, parts of Africa.
- Zone IVa (Hot-Humid/Tropical): 30°C / 65% RH - Southeast Asia, India, Brazil.
- Zone IVb (Hot/Higher Humidity): 30°C / 75% RH - Coastal tropics, like parts of Indonesia and the Caribbean.
Testing Intervals: When Do You Check?
You don’t just test once. You test at intervals: 0, 3, 6, 9, 12, 18, 24, and 36 months. The early points (3 and 6 months) are critical. That’s when most degradation shows up. Why so often? Because some drugs break down fast. A 2022 study by the American Association of Pharmaceutical Scientists found that 62% of failures in solid oral tablets came from humidity cycling-not constant exposure. A pill sitting in a bathroom cabinet gets wet, dries out, gets wet again. Standard tests don’t always simulate that. That’s why labs now use dual-loop humidity systems to keep conditions stable within ±2% RH, not ±8%.What Counts as a ‘Significant Change’?
This is where things get messy. ICH Q1A(R2) says a drug has failed if there’s a “significant change” in any key quality attribute. But it doesn’t define “significant.” In practice, that means:- A 5% drop in active ingredient concentration (e.g., from 100% to 95%)
- A 1% increase in impurities above the ICH qualification threshold
- A visible change in color, texture, or dissolution rate

Real-World Failures and Lessons Learned
It’s not theoretical. Failures happen. In 2021, Teva had to recall 150,000 vials of Copaxone® because their stability tests didn’t catch protein aggregation at 40°C. The drug was meant to treat multiple sclerosis. The failure was traced to a single step in the manufacturing process that changed how the protein folded under heat. Merck, on the other hand, used intermediate testing to catch a polymorphic shift in Keytruda®-a cancer drug. Without that test, the drug might have worked fine in the U.S. but lost potency in Brazil. They fixed it before launch. And it’s not just big pharma. A 2023 LinkedIn poll of 142 stability professionals found that 78% had experienced a temperature excursion in their chambers-sometimes over ±2°C. That’s enough to invalidate a 12-month study. One company lost six months of data and a $300,000 investment because a power surge spiked the lab’s fridge to 12°C for eight hours.What’s Changing? The Future of Stability Testing
The ICH Q1A(R2) standard is 20 years old. It was built for pills and syrups-not mRNA vaccines or antibody-drug conjugates. New tools are emerging:- Predictive modeling: Companies are using high-temperature stress tests (up to 80°C) to predict degradation in weeks, not years. 74% of top 20 drugmakers now use this. But regulators are skeptical. The EMA rejected 8 model-based submissions in 2022-2023.
- Real-time testing: The FDA is piloting Process Analytical Technology (PAT) for drugs made with continuous manufacturing. This could cut stability testing time by 30-50%.
- Dynamic humidity testing: Instead of fixed 60% RH, labs are now testing with cycling humidity-mimicking real storage conditions.
What You Need to Do
If you’re developing a drug:- Match your long-term test to your target market’s climate zone.
- Run accelerated testing at 40°C/75% RH for six months-even if your product is refrigerated.
- For cold-chain drugs, use the refrigerated protocol: 5°C long-term, 25°C accelerated.
- Test at 0, 3, 6, 9, 12, 18, 24, and 36 months. Don’t skip early points.
- Document every chamber’s temperature and humidity log. ±0.5°C and ±2% RH are the minimum.
- Prepare for regulator pushback on “significant change.” Have statistical analysis ready.
What are the standard temperature and humidity conditions for long-term stability testing under ICH Q1A(R2)?
ICH Q1A(R2) allows two options for long-term stability testing: 25°C ± 2°C at 60% RH ± 5% RH, or 30°C ± 2°C at 65% RH ± 5% RH. The choice depends on the climatic zone of the target market. For example, products sold in tropical regions (Zone IVa) must use the 30°C/65% RH condition. At least 12 months of data is required for U.S. regulatory submissions.
Why is accelerated testing done at 40°C and 75% RH?
The 40°C/75% RH condition is designed to simulate extreme environmental stress-not normal storage. It’s a predictive tool. For most small-molecule drugs, six months at these conditions correlates to about two years of real-time stability at 25°C/60% RH. It helps identify degradation pathways early so formulation issues can be fixed before launch.
Do refrigerated drugs follow the same stability rules as room-temperature drugs?
No. Refrigerated drugs are tested at 5°C ± 3°C for long-term stability and 25°C ± 2°C at 60% RH ± 5% RH for accelerated testing. This reflects their real-world risks-like temperature excursions during transport-rather than heat degradation. Using 40°C for refrigerated products would not be scientifically valid.
What is the purpose of intermediate testing in stability studies?
Intermediate testing (30°C/65% RH for 6 months) is required only when long-term testing is done at 25°C and accelerated testing shows a significant change. It bridges the gap between room-temperature and high-stress conditions, helping to confirm whether degradation is real or just a result of extreme testing. It’s a safeguard, especially for drugs destined for hot-humid markets.
How do climatic zones affect stability testing requirements?
The ICH defines five global climatic zones, each with specific temperature and humidity targets. For example, Zone IVa (tropical) requires 30°C/65% RH, while Zone IVb (higher humidity) requires 30°C/75% RH. If your drug is marketed in Zone IV, you must generate stability data under those conditions-even if your main market is temperate. Failing to do so can lead to product rejection or recalls in those regions.
What happens if a stability study fails?
A failed stability study can trigger regulatory action. The FDA may issue a warning letter, delay approval, or require a product recall. In 2021, Teva recalled 150,000 vials of Copaxone® after stability tests revealed protein aggregation at 40°C. Companies often spend months re-formulating or re-testing, which delays launch and costs hundreds of thousands of dollars.

Sangeeta Isaac
January 21, 2026so like... i just threw my insulin in the backseat of my car for 3 hours and it's still fine? 🤔 guess i've been doing stability testing wrong my whole life
Samuel Mendoza
January 22, 202640°C? That’s not stress testing. That’s arson. If your drug can’t survive 40°C, it shouldn’t be on the market. Period.
Stephen Rock
January 23, 2026the fact that regulators don’t define 'significant change' is the entire joke
one guy says 4.8% is fine another says it’s a national emergency
we’re not制药 engineers we’re astrology judges
shubham rathee
January 25, 2026in india we just leave pills in sun for 2 weeks and if they dont melt its good enough
why pay for fancy labs when god already tested it
Malvina Tomja
January 26, 2026Let me be clear: the ICH Q1A(R2) guidelines are not suggestions. They are the minimum threshold of scientific integrity. Any deviation-especially in Zone IV environments-represents a failure of corporate responsibility. The fact that companies still cut corners on humidity control is not just negligent, it’s criminal. The FDA’s 27 warning letters in 2022? That’s the tip of the iceberg. And don’t get me started on how some labs still use ±8% RH tolerance. That’s not science. That’s a dare.
There is no excuse for not implementing dual-loop humidity systems. Not in 2024. Not ever. The 2022 AAPS study proved humidity cycling causes 62% of tablet failures. That’s not noise. That’s a systemic blind spot. And yet, half the labs I’ve audited still treat it like a suggestion.
And don’t tell me about cost. The $300,000 loss from an 8-hour power spike? That’s pocket change compared to a recall. A single vial of Copaxone® recalled because of protein aggregation? That’s not a manufacturing error. That’s a leadership failure.
Every time a company says ‘we followed the letter of the guideline’ while ignoring the spirit, they’re gambling with lives. And the people who pay the price aren’t shareholders. They’re the diabetic in Mumbai. The cancer patient in São Paulo. The child in Jakarta with a vaccine that never worked.
It’s not about compliance. It’s about honor. If you can’t guarantee that your drug won’t degrade in a tropical warehouse, you don’t deserve to make it.
And if you’re still using 25°C data for Zone IVb? You’re not just outdated. You’re dangerous.
Melanie Pearson
January 27, 2026Let’s be honest-this whole system was designed for Western markets. Zone IVb requirements are an afterthought. The WHO added refrigerated protocols in 2018 because people kept dying in the Global South. But pharma still treats tropical regions like an inconvenience, not a market. We don’t need more guidelines. We need enforcement. And accountability. And yes, punishment.
When a company loses six months of data because their fridge spiked to 12°C? That’s not a ‘mistake.’ That’s incompetence. And incompetence should cost them their license. Not a slap on the wrist and a ‘please do better.’
Why are we still using 20-year-old standards for mRNA vaccines? Because legacy systems don’t die-they just keep poisoning innovation. The EMA rejected 8 model-based submissions? Good. Let them prove it in real time. Not in a lab with perfect conditions. In a truck on a road in Lagos.
This isn’t science. It’s colonialism with a lab coat.
MARILYN ONEILL
January 27, 2026so like... the pill just needs to not turn into slime right? why are we overcomplicating this
also why is everyone so mad about 4.8% drop
it's like 1/20 of a pill
who even notices
Alex Carletti Gouvea
January 29, 2026if you're selling in India or Brazil you better be testing at 30/65 or 30/75
no excuses
the FDA doesn't care what your US data says
if your drug melts in the sun you're not a pharma company
you're a liability