Pharmacovigilance: Understanding Drug Safety, Side Effects, and Real-World Risks
When you take a pill, you’re trusting that it’s been tested for safety—but what happens after it’s on the market? That’s where pharmacovigilance, the science of monitoring drug safety in real-world use. It’s not just about clinical trials; it’s about spotting problems that only show up when thousands of people use a drug daily. This includes everything from rare heart rhythms triggered by antibiotics to dizziness from blood pressure meds, or even confusion caused by mixing alcohol with antidepressants. Pharmacovigilance isn’t a backroom process—it’s your protection.
It’s also why some people react differently to generic medications, cheaper versions of brand-name drugs that must meet bioequivalence standards. While they’re supposed to work the same, tiny differences in fillers or how the body absorbs them can matter—especially with drugs like levothyroxine or warfarin. These are high-risk medications where even small changes can cause serious harm. That’s why pharmacovigilance tracks these cases closely, and why your doctor might ask you to stick with the same brand or batch. It’s not about distrust—it’s about precision. The same goes for drug interactions, when one medication or substance changes how another works in your body. Alcohol with painkillers? Dangerous. Vitamin K with blood thinners? Can undo the effect. Even herbal supplements like Brahmi or saw palmetto can interfere. Pharmacovigilance collects these real-life stories to warn others before more people get hurt.
Some side effects are obvious—nausea, sweating, dizziness. Others sneak up: a drop in sodium from SSRIs in older adults, or a QT prolongation from azithromycin that can trigger a fatal heart rhythm. Pharmacovigilance connects the dots between symptoms and meds, even when the link isn’t obvious. That’s why keeping a simple medication list with safety alerts matters—it turns you into part of the system. You notice the dizziness when you stand up. You track the bleeding after a cut. You report the weird rash. Those reports feed into global safety databases.
There’s no magic shield against drug risks—but pharmacovigilance is the closest thing we have. It’s the reason you’re told to avoid grapefruit with certain pills, why your thyroid dose gets checked every few months, and why some drugs get black box warnings. Below, you’ll find real, practical guides on the most common and dangerous side effects, how to spot them early, and what to do before it’s too late. This isn’t theory. It’s what happens when people take meds—and what you need to know to stay safe.
The Science of Medication Safety: Understanding Risk, Benefit, and Real-World Evidence
Medication safety isn't just about avoiding mistakes - it's science that tracks real-world drug risks using massive data, AI, and real-life evidence to protect patients from hidden dangers.
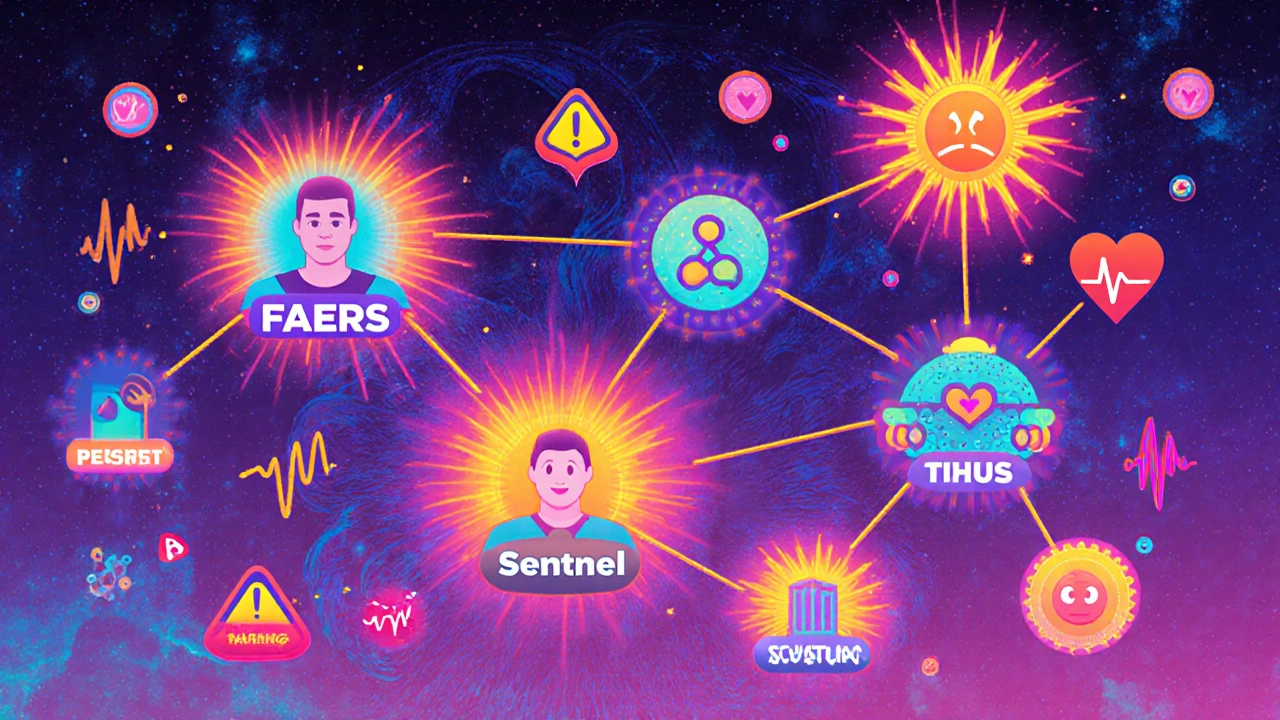
How to Track Post-Marketing Studies for Drug Safety
Learn how to effectively track post-marketing drug safety studies using FAERS, Sentinel, and real-world data. Understand key systems, common delays, regulatory actions, and emerging tools to protect public health.